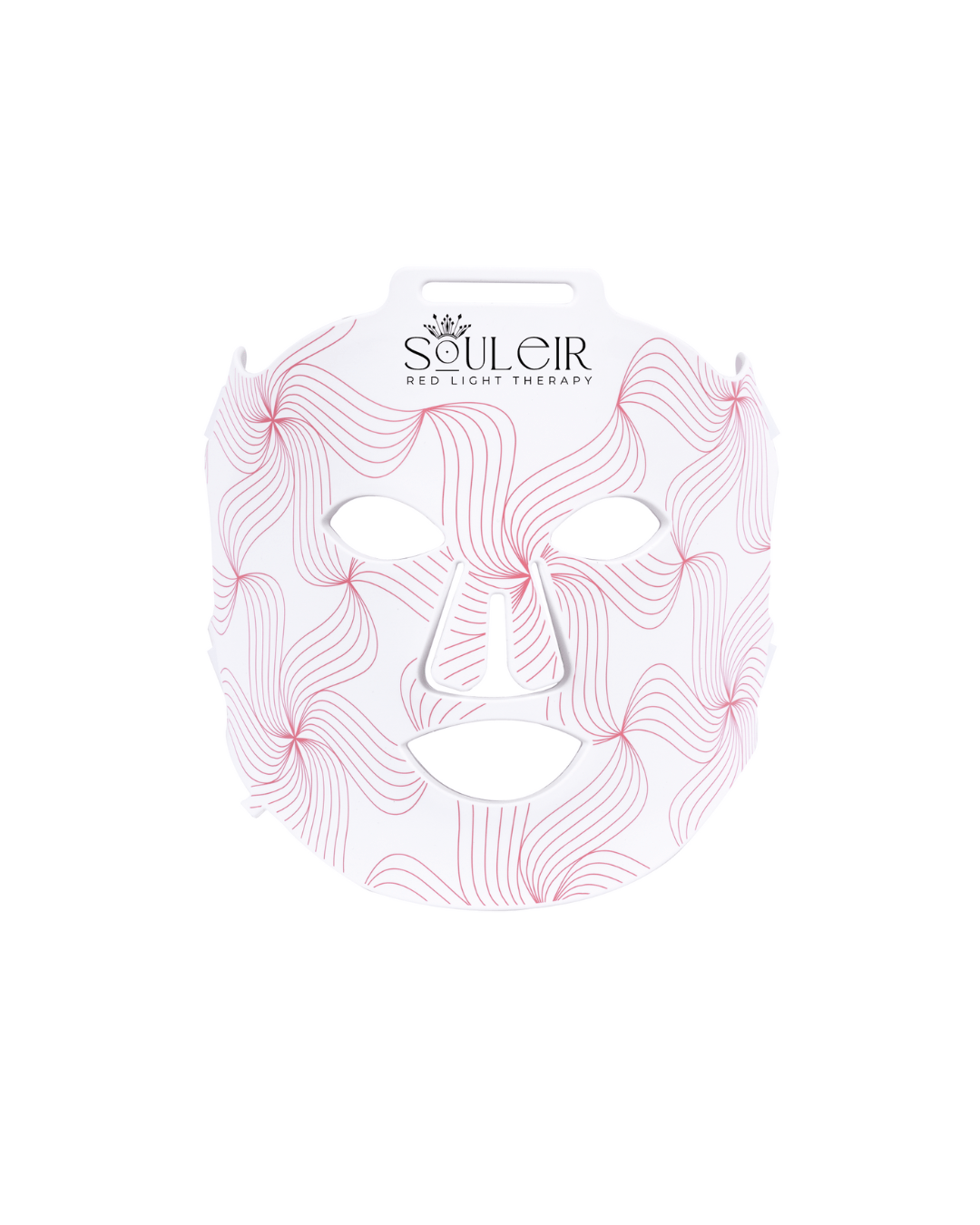




Youth Restore LED Therapy Mask
60 Day money-back guarantee
FREE Delivery
Product Description
Souleir's most advanced LED light therapy mask for radiant, healthier-looking skin:
- Visibly softens the look of fine lines + promotes a brighter, more refreshed complexion
- Supports natural collagen production + skin elasticity
- Soothes irritated skin + reduces redness
- Suitable for sensitive skin
LED essential benefits
- Boosts collagen an+ elastin for firmer skin
- Fades pigmentation, rosacea, + sun damage
- Softens fine lines + wrinkles
- Soothes eczema, psoriasis, + acne
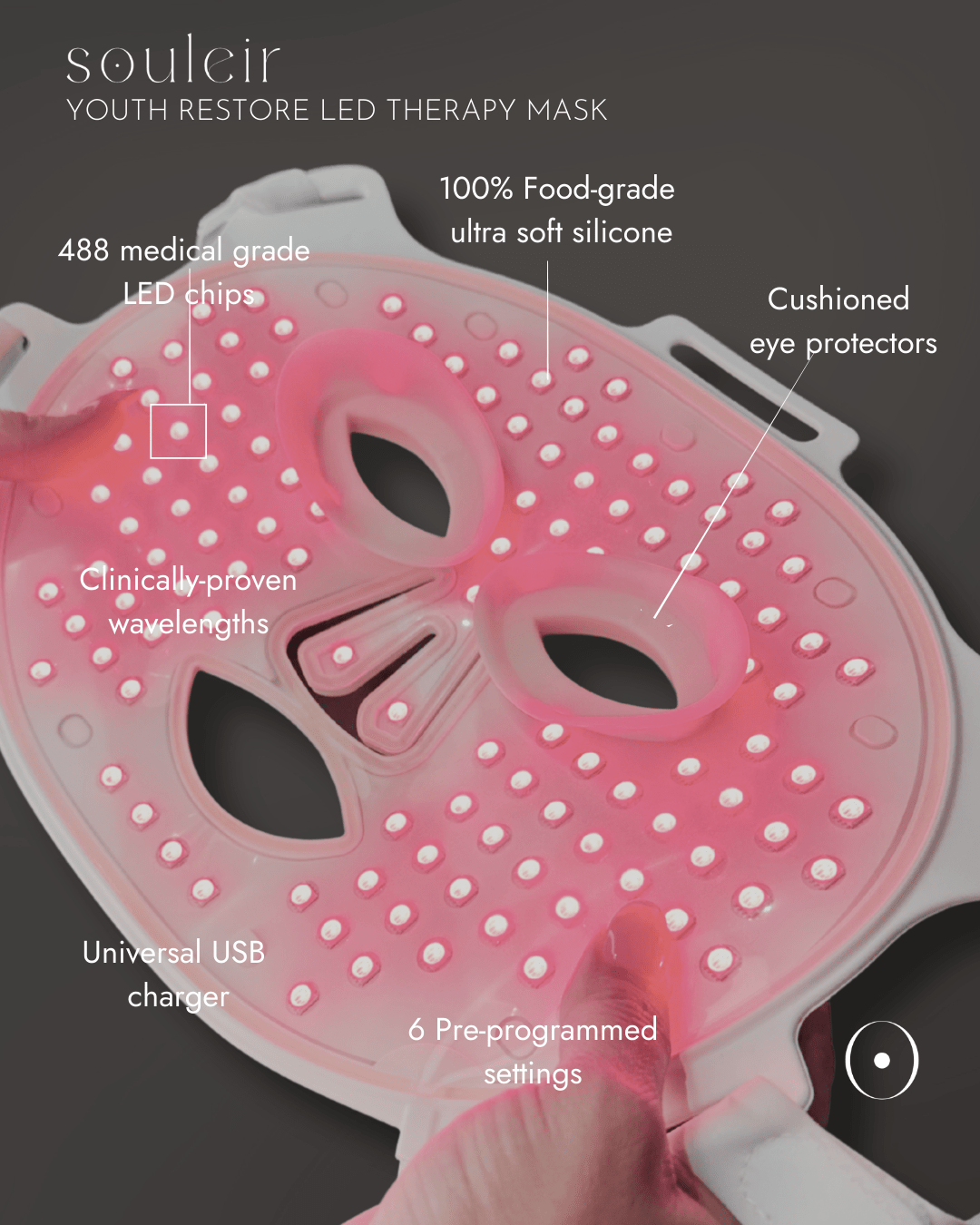
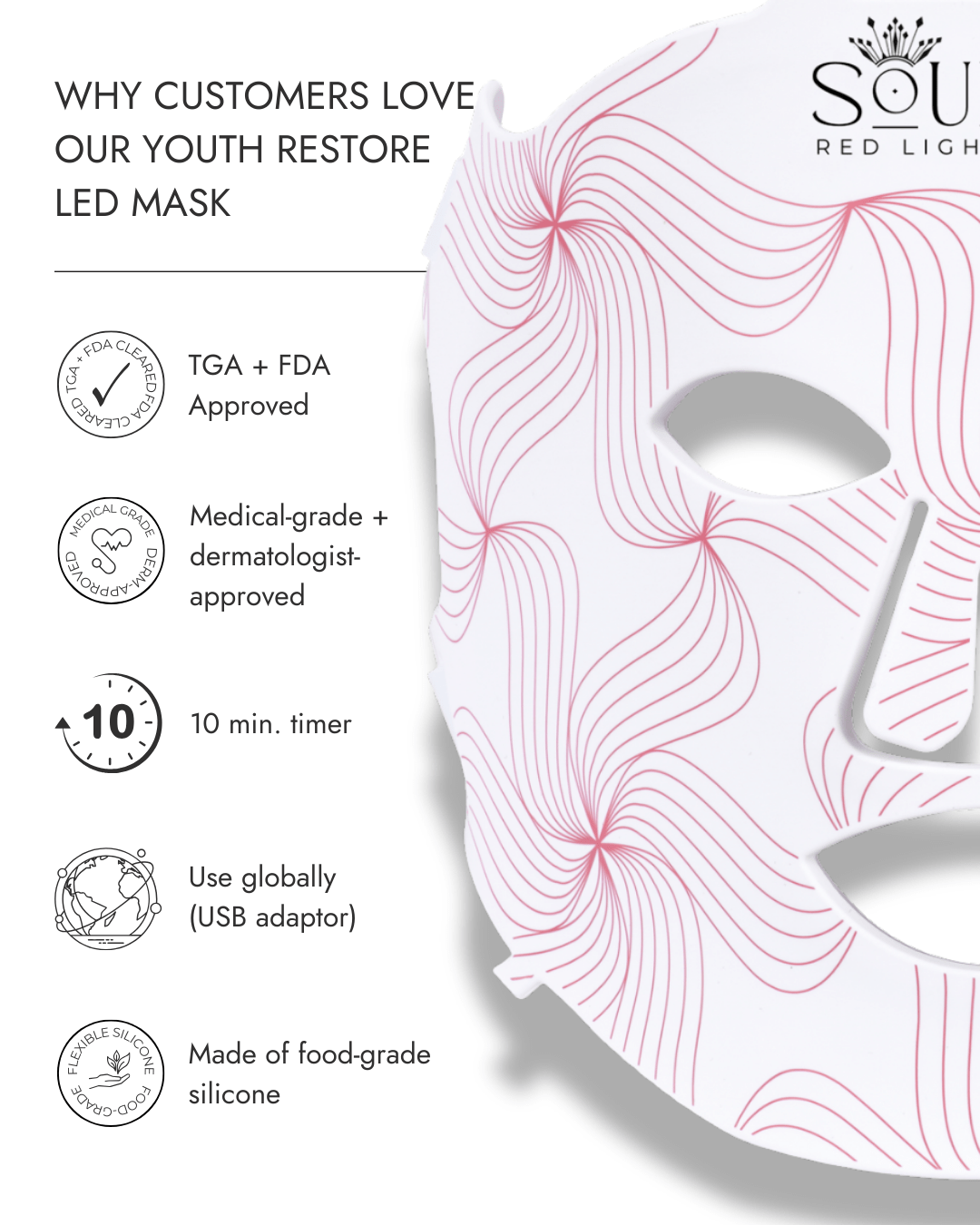
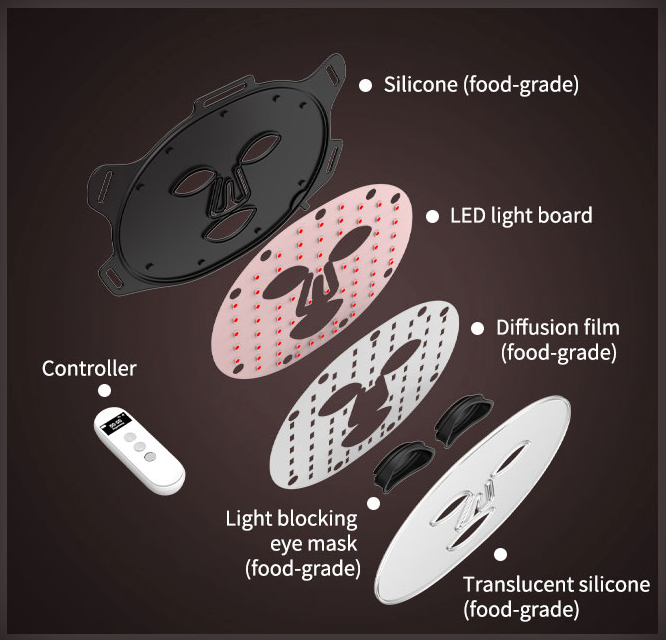
- Made with food-grade, BPA-free silicone
- Safe for all skin types, even sensitive skin
- Professional results at home
4-in-1 LED bead technology
Our LED mask features 4 chips in a single light bead, offering a powerful, compact design that outperforms traditional single-chip masks.
This setup ensures even light distribution, tackling multiple skin concerns at once—reducing inflammation + boosting collagen + killing acne bacteria + promoting healing.
With fewer components, the design is sleek, energy-efficient, + delivers maximum effectiveness.
Product specifications
4 Clinically Recognised Wavelengths
LED Face Mask Colours
- 415 nm (Blue): Targets acne + bacteria
- 590 nm (Yellow): Reduces inflammation + improves skin texture
- 660 nm (Red): Boosts collagen + enhances elasticity
- 850 nm (Near-Infrared): Promotes skin regeneration + healing
Number of LEDs:
FACE MASK:
488 LEDs - the most on the market
– Advanced 4-in-1 Technology for Maximum Results.
The Souleir Youth Restore LED Therapy Mask is equipped with 122 LED bulbs, each utilising cutting-edge 4-in-1 LED chip technology, delivering a total of 488 LEDs - the highest qty on the market.
With an irradiance of 30-35 mW/cm², this advanced design ensures optimal light penetration + comprehensive skin coverage, targeting your skin’s unique needs for visible, radiant results.
Optimal Intensity:
Avoids excessive power to prevent photochemical stress, ensuring maximum effectiveness
Photobiological Optimality:
Prioritises wavelength specificity over raw power to stimulate mitochondrial cytochrome c oxidase for sustained cellular response and minimised risk of saturation
Product Specifications:
Face Piece Dimensions:
310*207*4.5mm
Weight:
0.38kg
USB:
Type-C
Input Voltage:
5V 2A
Battery Capacity:
5000mAh (7-10 uses)
Functionality:
6 skincare modes, brightness adjustment, pulse function
Pre-programmed modes & functionality
Mode 1: Repairing 660nm
Mode 2: Rejuvenation 590nm + 660nm
Mode 3: Anti-Aging 660nm + 850nm
Mode 4: Morning Skincare 590nm + 660nm + 850nm
Mode 5: Anti-Acne 660nm + 850nm
Mode 6: Bedtime Skincare 590nm + 660nm + 850nm + 415nm
LED Face Mask Colours
1. Blue light Sterilises acne producing bacteria, reduces inflammation + scars, + increases skin oxygen.
2. Yellow Light Balances skin texture, stimulates red blood cells, reduces redness, swelling, + fine lines.
3. Red Light Anti-aging, promoting collagen, tightening + increasing elasticity.
4. Near-infrared (NIR) light Skin repair, improve blood circulation, + increase skin moisture.
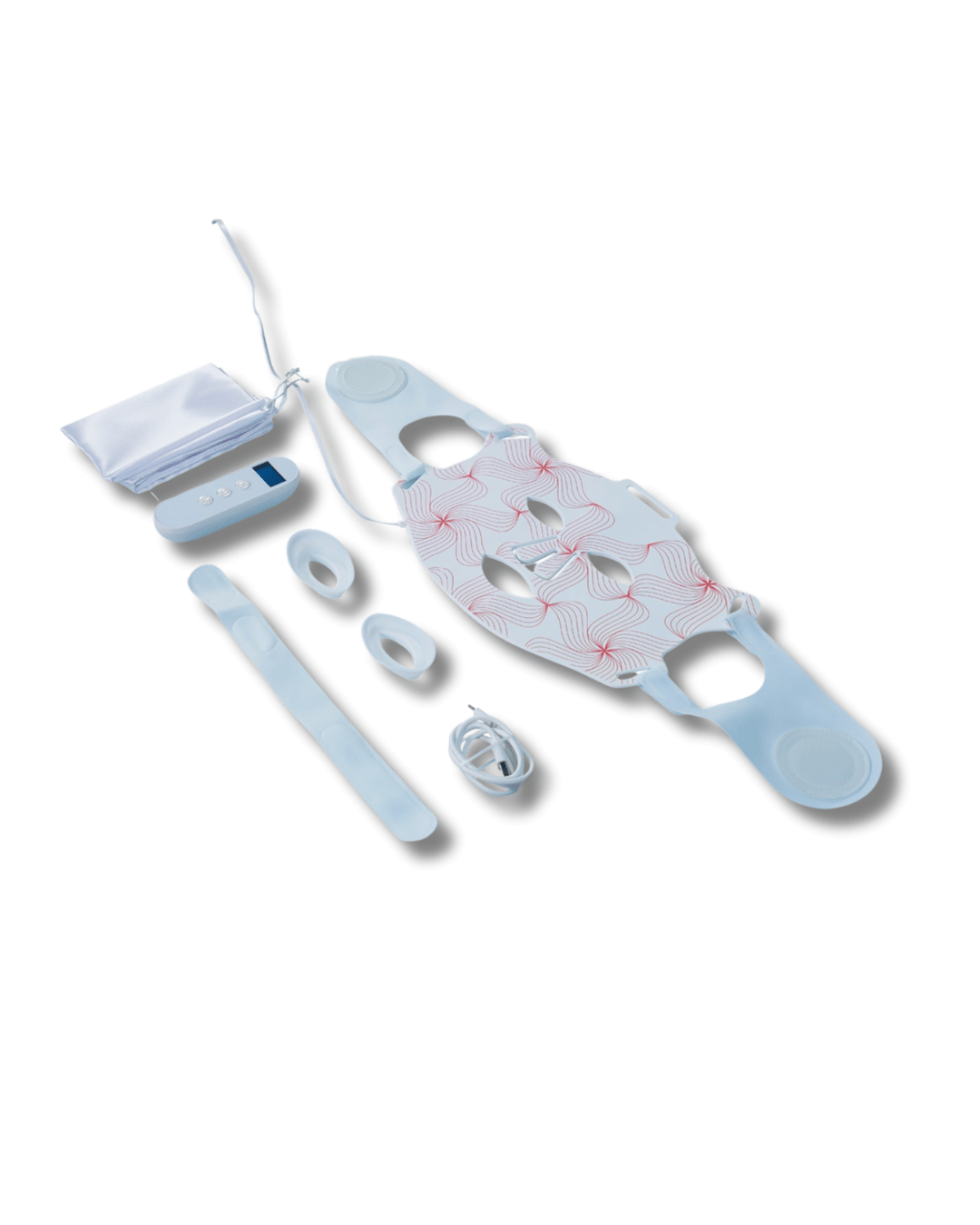
What you'll receive
- 1 x LED Device
- Velcro fastener attachments
- 1 x pair of eye covers
- 1 x USB cable
- 1 x Remote device
- 1 x Drawstring dust bag for storage
- 1 x Instruction Manual
Certifications + compliance
- TGA Approved
- IEC 60335-2-115:2021
- IEC 60335-1:2010
- IEC 60335-1:2010/AMD1:2013
- IEC 60335-1:2010/AMD2:2016
- AS/NZS 60335.2.115:2021+A1:2022
- AS/NZS 60335.1:2020+A1:2021
- SAA
- ISO13485
- FDA
- ROHS
- MDSAP
- FCC
- CE
Choose options















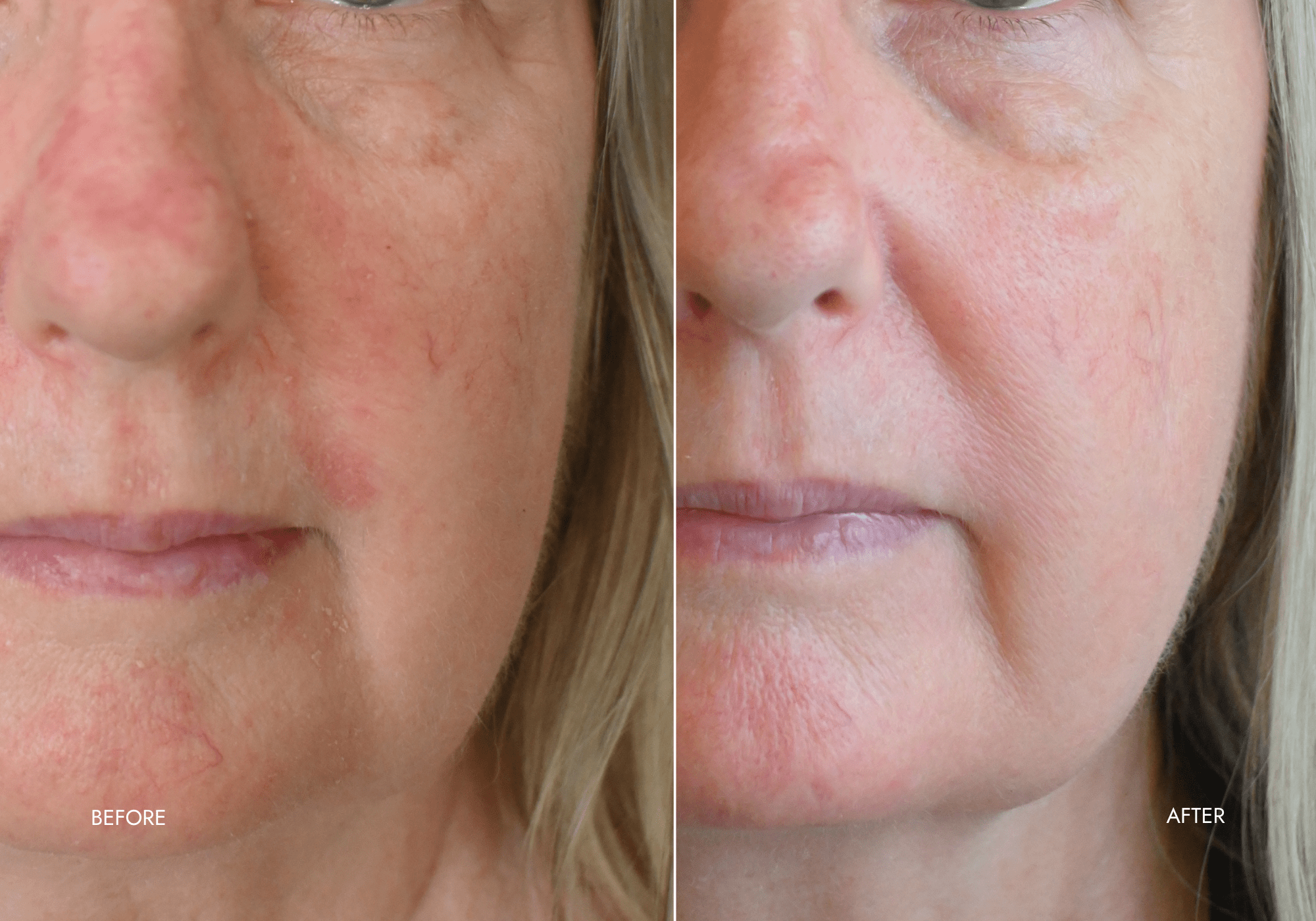
Client Results
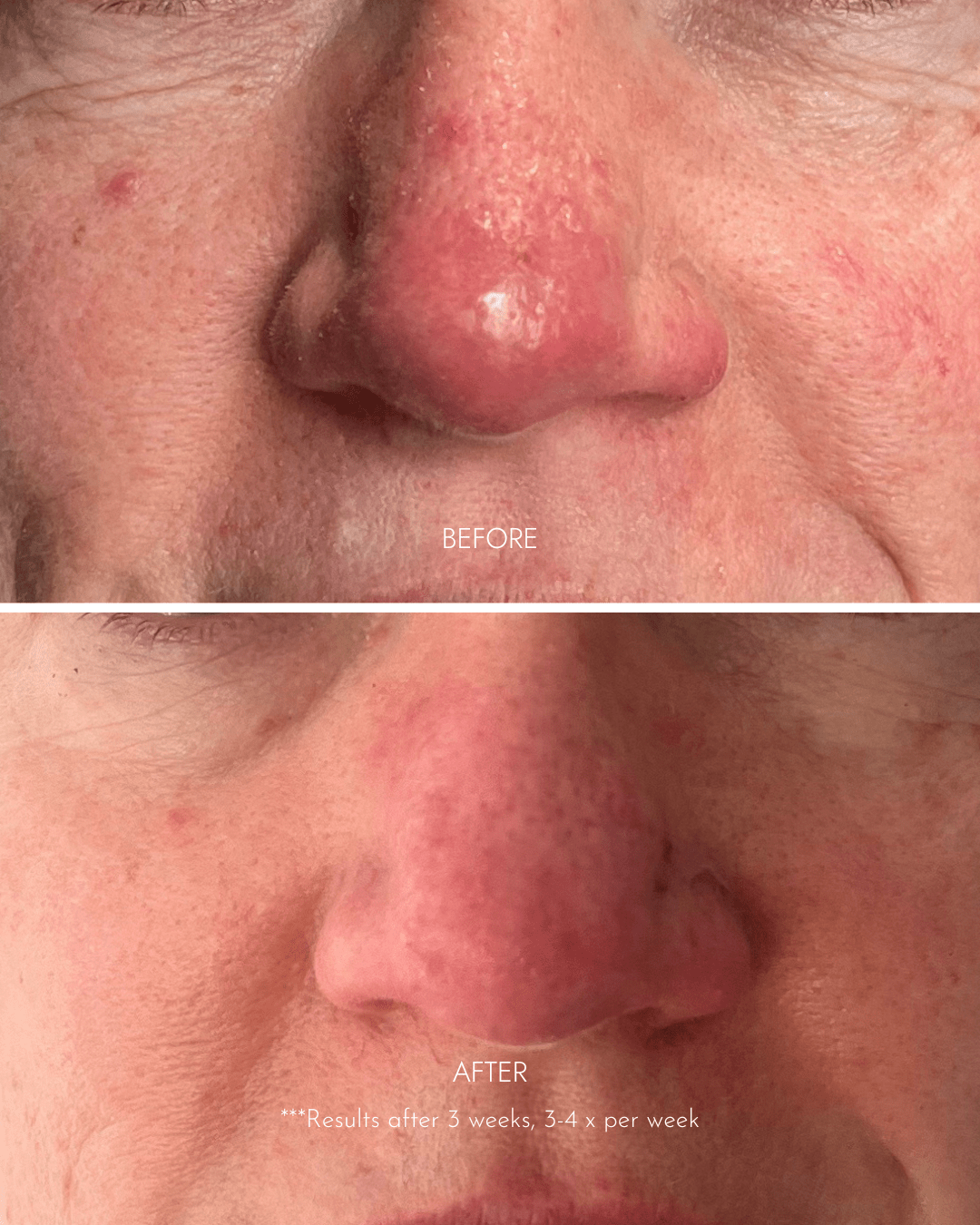
"Noticable results within 3 days!"
- Soothed red flaky + scaly skin
- Red patches diminished
- Skin is now more hydrated
- Smoother + softer in texture
- Healthier + more luminous in appearance
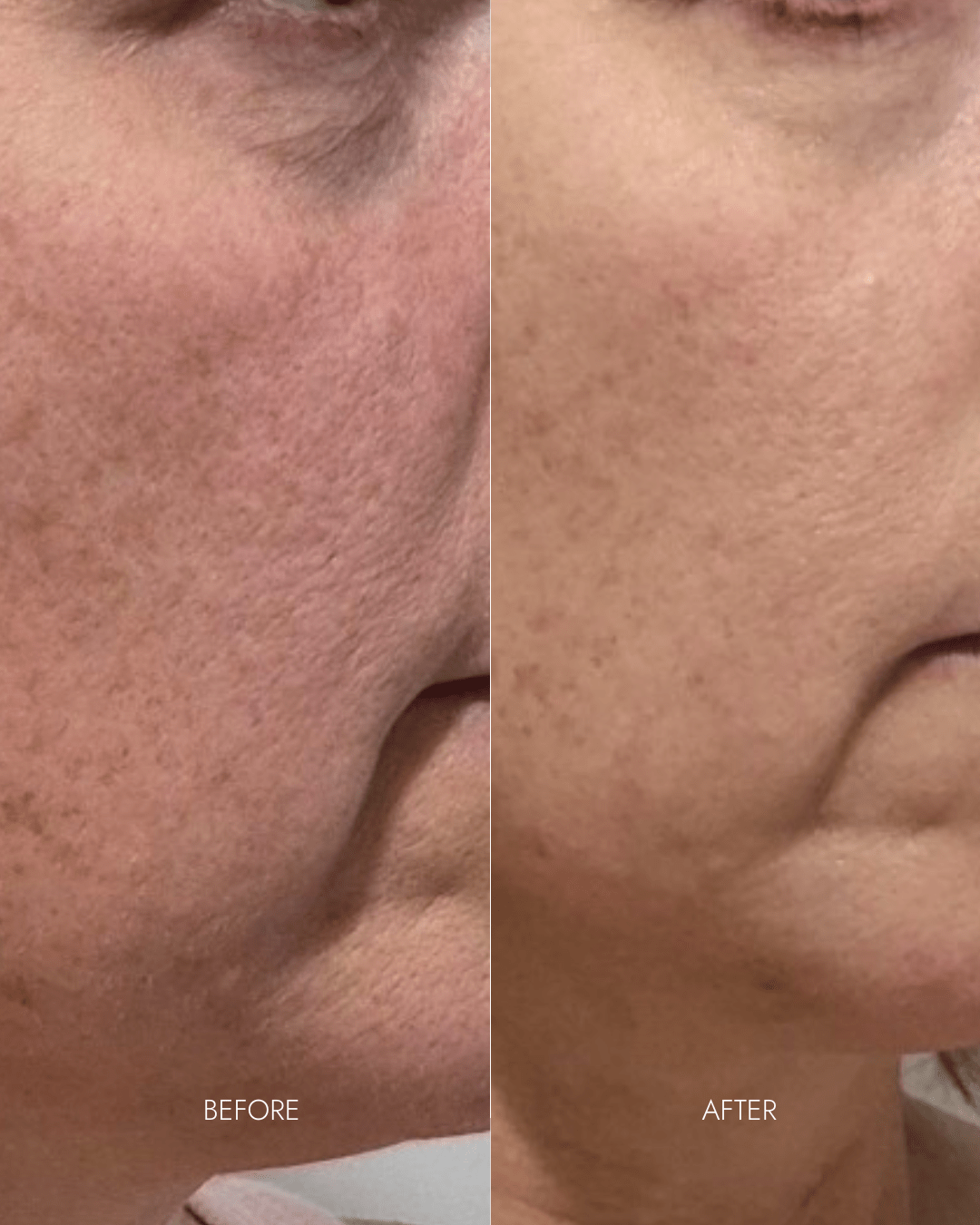
"I had spent thousands of dollars trying other products that didn't work. My skin would go back to being red with dry patches just 2 days after getting facials at salons."
- Lisa C. (Practice Manager, GP medical centre)
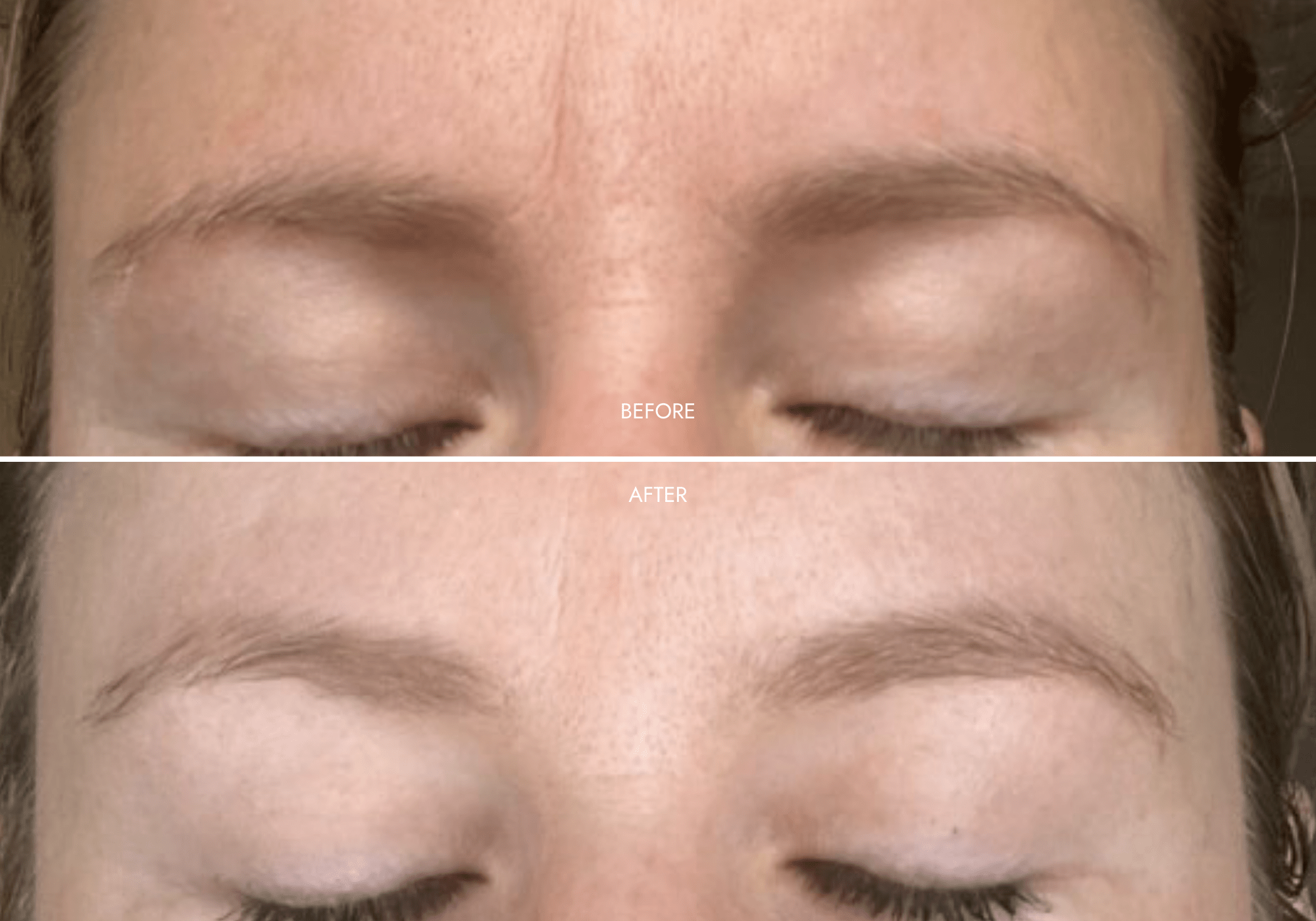
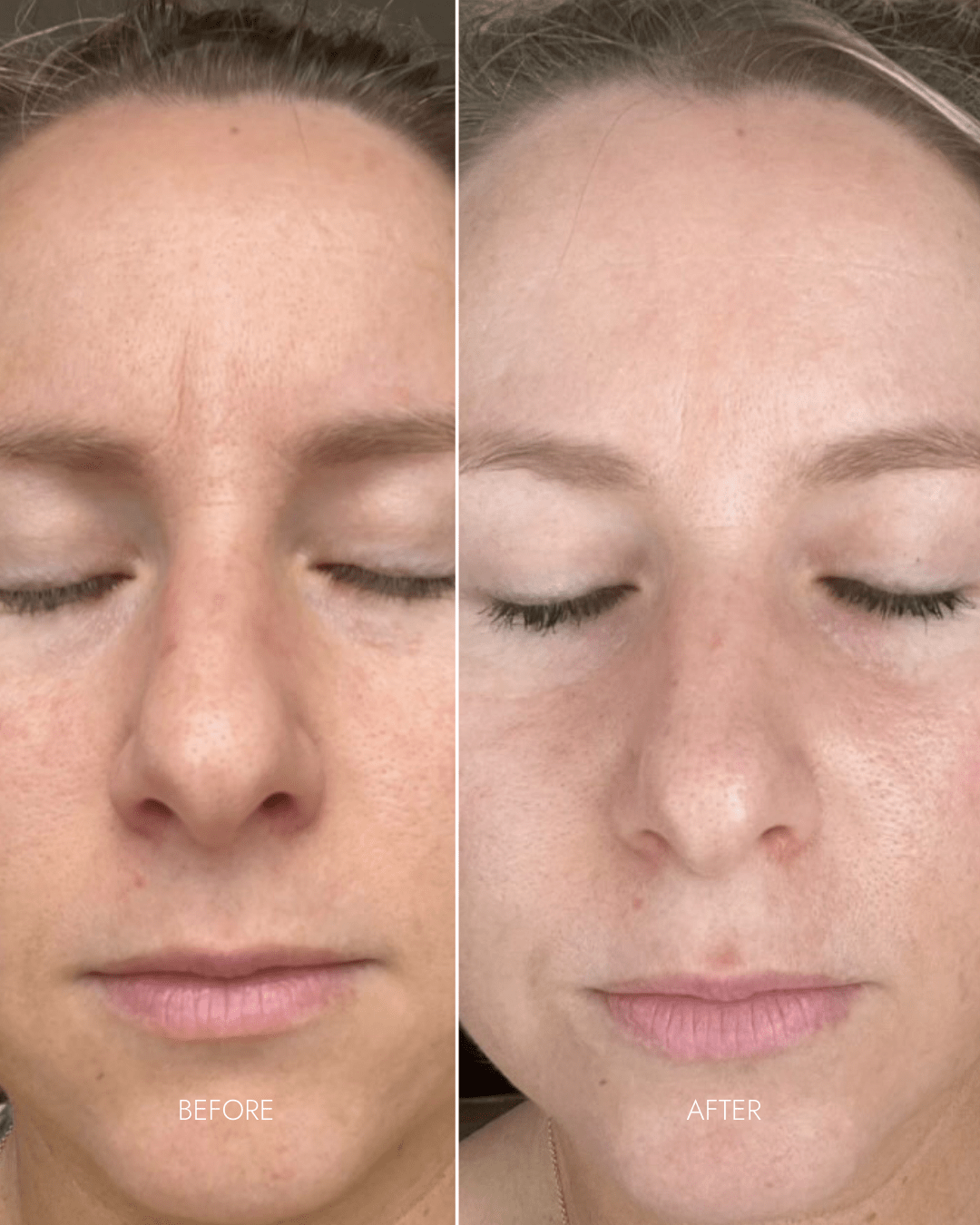
Client Results
"Amazing Product!"
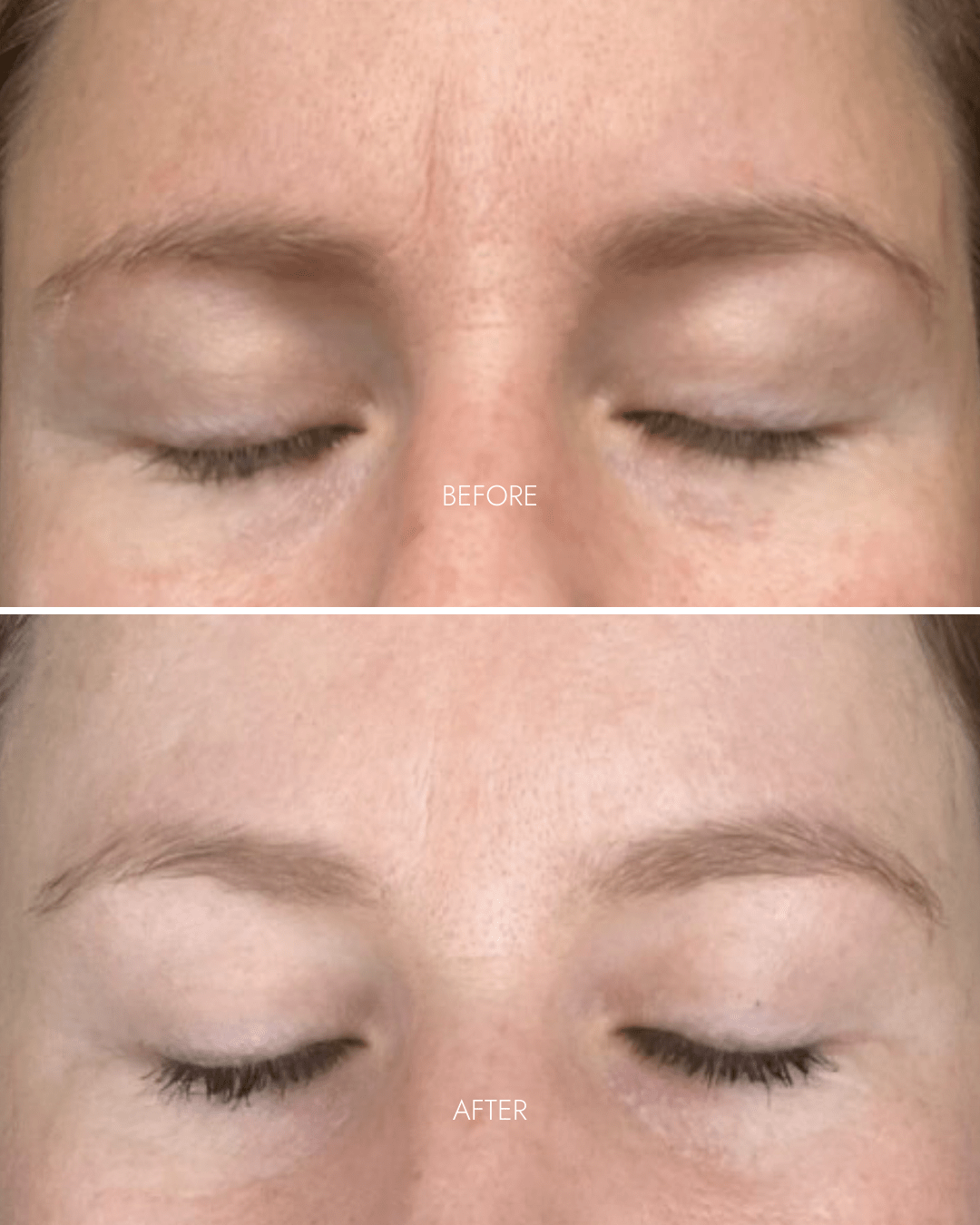
- Reduced wrinkles in 11s area
- Lighter + brighter skin-tone
- Reduced redness + pigmentation
- Smoother skin + refined pores
"Amazing customer service to start off with!! All my questions and queries are answered in a super timely manner. Love the product. Can’t fault it! Lots of different settings and was very easy to set up. I find it very relaxing popping the face mask on for 10 minutes whilst I’m laying in bed. Feels good to invest in my skin care. Highly recommend"
- Michelle G. (Nurse)
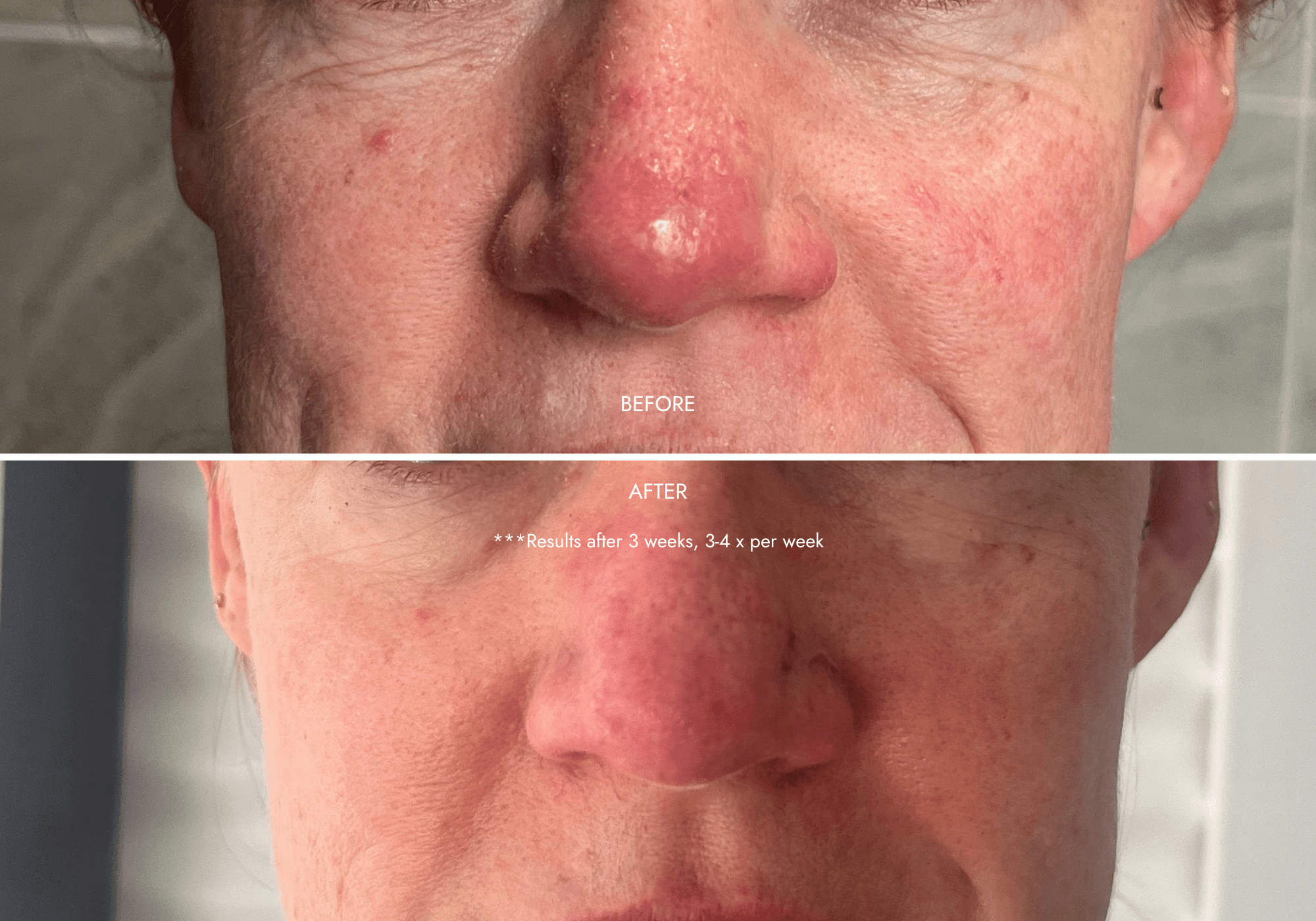
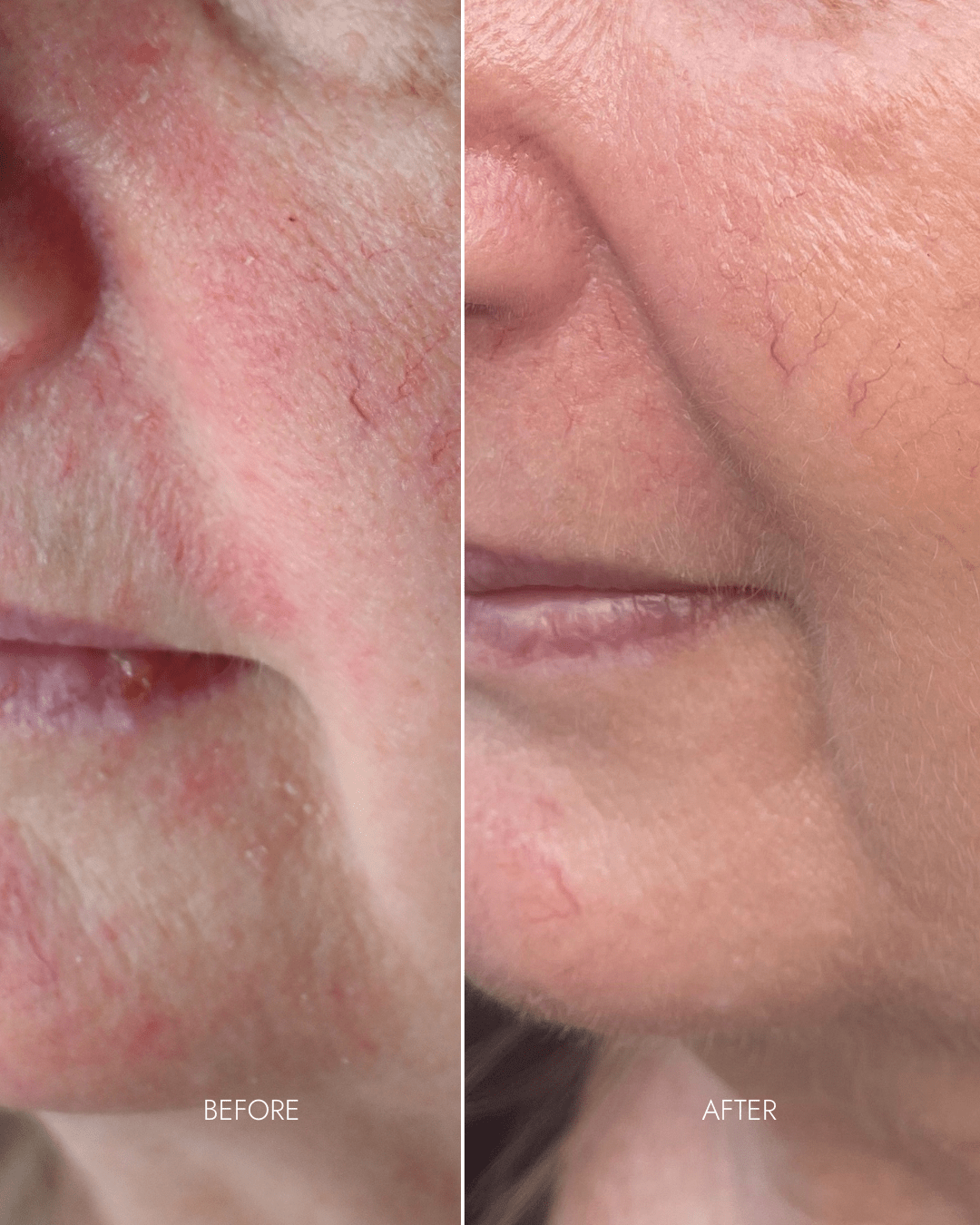
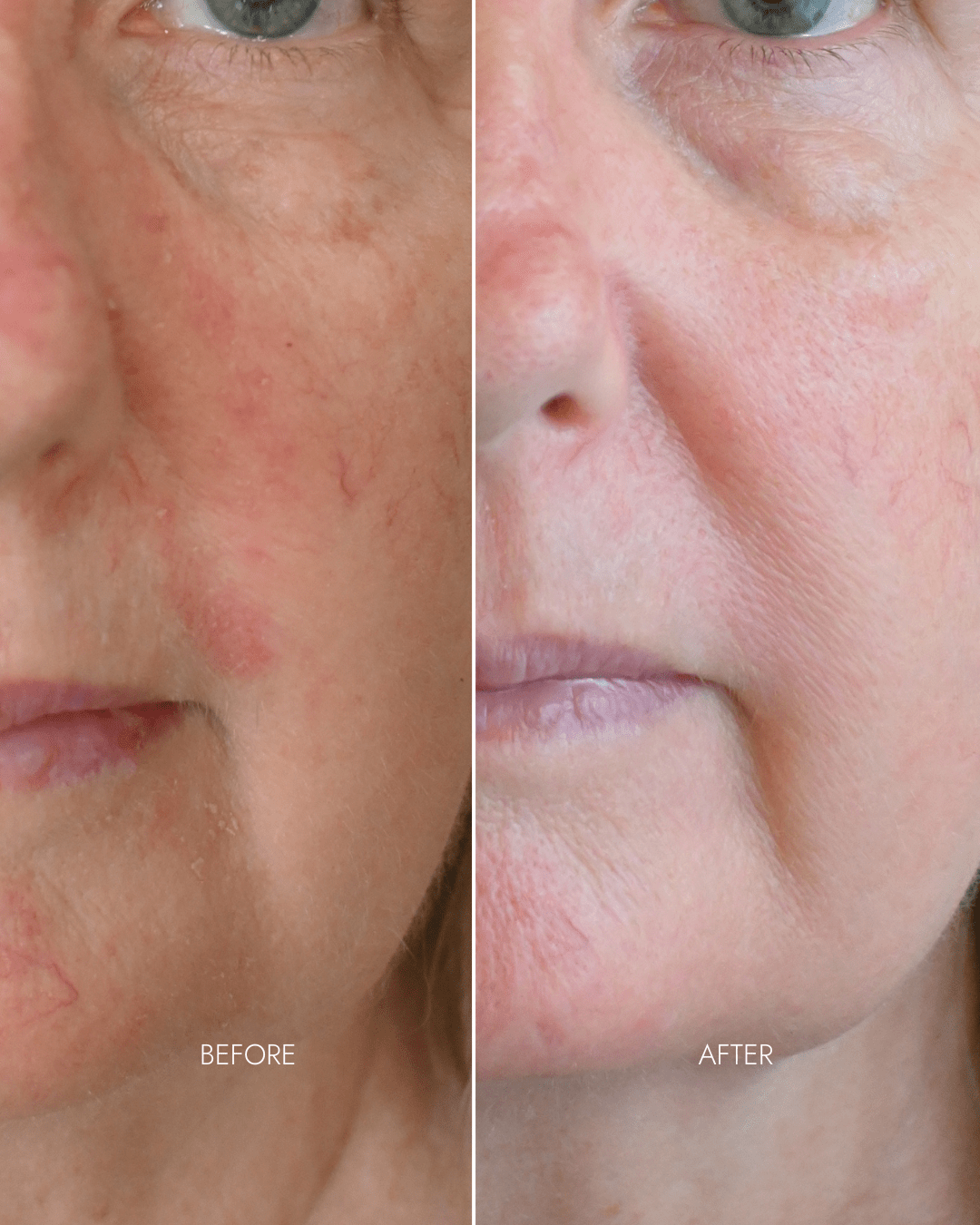
Client results
"Noticed instant difference in texture"
- Redness/rosacea reduced despite minimum usage (3 x per week)
- Smoother skin texture
- Refined pores
- Fine lines diminished under eyes
"... My main concern was improving my skin texture. As a rosacea sufferer, I had noticed an increase in unevenness that I just couldn’t get a handle on with my skincare. Once I started using the youth restore system I noticed an immediate change in the texture and also a calmness in my skin - it has only continued to improve the more I have used the mask."
- Hilary
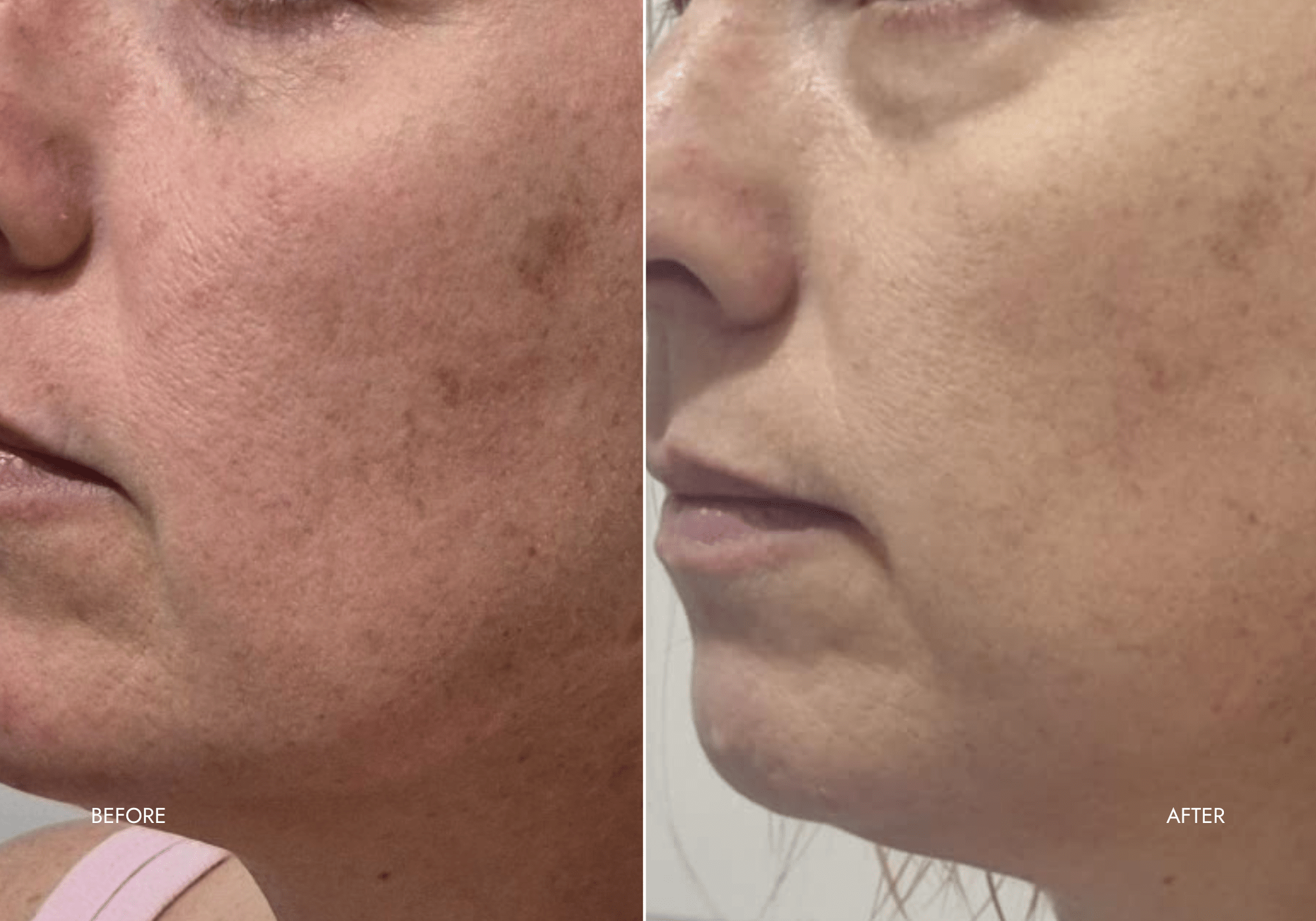
Client Results
"Very fast results!"
- Improved elasticity
- Firmer skin
- Reduced fine lines + pores
- Even + brighter skin-tone
- Reduced sun damage + pigmentation
- Smoother skin
- Brighter complexion
"Very fast results! I'm a 46 y.o female with oily, pimple-prone skin, and I spent too much time tanning as a teen. I purchased the face mask to deal with my sun damage, pigmentation and big pores. I used the 'anti-aging' setting for 2 weeks, and although hard to believe, I noticed a fresher, refined look after the first couple of uses. Even my teenage son commented, "Is that you without make-up on? Wow." I'm obsessed and now I use the mask as often as suggested. Thanks Souleir!"
- Taryn W. (Business Owner)
Trusted by those who expect more
Refined innovation. visible results.
See how the Souleir Youth Restore LED Mask is delivering a new standard in skin rejuvenation through the stories of those who've experienced it firsthand.
Scientifically engineered.
Expertly crafted.
Remarkable results.
Souleir LED Therapy
Proven To Diminish the Signs of Aging
We partner with manufacturers with 15+ years of LED expertise and 100+ innovative products.


Advanced Technology
#1
LEDs with latest 4-in-1 LED chip beads
Medical-grade, Clinically validated wavelengths
Backed by 10+ years of research

Clinical Results
#2
25% boost in skin luminosity
35.2% increase in collagen production
23.9% reduction in wrinkles
Firmer, more radiant skin

Turn your daily regimen into a powerful skincare ritual
#3
Integrate with your existing skincare routine – our mask amplifies the effectiveness of your favourite products.

Clinically Proven. Dermatologist Recommended.
#4
Experience professional-grade skin rejuvenation from the comfort of your home.